Testosterone Treatment in Adult Men With Age-Related Low Testosterone: A Clinical Guideline From the American College of Physicians
Amir Qaseem, M.D., Ph.D., MHA; Carrie A. Horwitch, M.D., MPH; Sandeep Vijan, M.D., MS; Itziar Etxeandia-Ikobaltzeta, Ph.D.; Devan Kansagara, M.D., MCR
Annals of Internal Medicine – January 7, 2020
Description:
The American College of Physicians (ACP) developed this guideline to provide clinical recommendations based on the current evidence of the benefits and harms of testosterone treatment in adult men with age-related low testosterone. This guideline is endorsed by the American Academy of Family Physicians.
Methods:
The ACP Clinical Guidelines Committee based these recommendations on a systematic review on the efficacy and safety of testosterone treatment in adult men with age-related low testosterone. Clinical outcomes were evaluated by using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) system and included sexual function, physical function, quality of life, energy and vitality, depression, cognition, serious adverse events, major adverse cardiovascular events, and other adverse events.
Target Audience and Patient Population:
The target audience includes all clinicians, and the target patient population includes adult men with age-related low testosterone.
Recommendation 1a:
ACP suggests that clinicians discuss whether to initiate testosterone treatment in men with age-related low testosterone with sexual dysfunction who want to improve sexual function (conditional recommendation; low-certainty evidence). The discussion should include the potential benefits, harms, costs, and patient’s preferences.
Recommendation 1b:
ACP suggests that clinicians should reevaluate symptoms within 12 months and periodically thereafter. Clinicians should discontinue testosterone treatment in men with age-related low testosterone with sexual dysfunction in whom there is no improvement in sexual function (conditional recommendation; low-certainty evidence).
Recommendation 1c:
ACP suggests that clinicians consider intramuscular rather than transdermal formulations when initiating testosterone treatment to improve sexual function in men with age-related low testosterone, as costs are considerably lower for the intramuscular formulation and clinical effectiveness and harms are similar.
Recommendation 2:
ACP suggests that clinicians not initiate testosterone treatment in men with age-related low testosterone to improve energy, vitality, physical function, or cognition (conditional recommendation; low-certainty evidence).
A gradual, age-associated decline in serum total testosterone levels begins in men in their mid-30s and continues at an average rate of 1.6% per year (1–5). This condition is referred to as age-related low testosterone and is accompanied by clinical symptoms associated with androgen deficiency. No well-defined, universally accepted threshold of testosterone levels exists below which symptoms of androgen deficiency and adverse health outcomes occur (6–8). The incidence of low testosterone in the United States is reported to be approximately 20% in men older than 60, 30% in those older than 70, and 50% in those older than 80 years (4), although the prevalence of syndromic low testosterone (defined as at least 3 sexual symptoms with a total testosterone level <11.1 nmol/L [320 ng/dL]) is lower (9). Uncertainty exists as to whether nonspecific signs and symptoms associated with age-related low testosterone, such as sexual dysfunction, decreases in energy and muscle mass, mood disturbances, changes in bone mineral density, cardiovascular disease, depression, decreased libido, erectile dysfunction, decreased volume of ejaculate, loss of body and facial hair, weakness, and mortality, are a consequence of age-related low testosterone or whether they are a result of other factors, such as chronic illnesses or concomitant medications (7, 10, 11).
The role of testosterone treatment in managing age-related low testosterone is controversial. The U.S. Food and Drug Administration (FDA) requires the pharmaceutical industry to label all testosterone medications to clearly state that their products are approved for use only in persons with low testosterone levels due to known causes (11).
Guideline Focus and Target Population
The purpose of this American College of Physicians (ACP) guideline is to present recommendations based on the best available evidence on the benefits, harms, and costs of testosterone treatment in adult men with age-related low testosterone. This guideline does not address screening or diagnosis of hypogonadism or monitoring of testosterone levels.
The target audience for this guideline includes all clinicians, and the target patient population includes adult men with age-related low testosterone. These recommendations are based on a systematic evidence review conducted by the Minnesota Evidence-based Practice Center and funded by ACP (12). This guideline is endorsed by the American Academy of Family Physicians.
Systematic Review of the Evidence
Additional details and methods for the supporting evidence review are included in the accompanying systematic evidence review paper (12) and in the Appendix. Reviewers searched several databases for studies and systematic reviews published in English from 1980 through May 2019. Reviewers assessed risk of bias by using standardized methods. A technical expert panel was convened to inform the evidence review and assist in refining the scope and key questions. In addition, ACP conducted a rapid review separate from the supporting evidence review to identify existing literature on patient values and preferences. The reviewer searched MEDLINE and PsycINFO from inception to November 2018, using a combination of keywords and MeSH (Medical Subject Headings) terms related to testosterone treatment and patient values and preferences (Supplement 1).
Main Outcomes
Clinical Guidelines Committee (CGC) members (clinicians and nonclinician public members) and CGC Public Panel members were asked a priori to independently rate the importance of evaluated outcomes. Quality of life; erectile function; cognitive function; and harms, including serious adverse events, major adverse cardiovascular events, deep venous thrombosis or pulmonary embolism, mortality, and prostate cancer, were rated as critical outcomes. Energy and vitality, physical function, mood (depression), fracture reduction, libido, and lower urinary tract symptoms were rated as important outcomes. All critical and important outcomes were considered in developing the recommendations. Sexual function included self-reported overall sexual function and erectile function and was measured by the Aging Males’ Symptoms (AMS) and International Index of Erectile Function (IIEF) scales. The IIEF domains include erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction; the AMS scale assesses disturbances in potency, decreased morning erections, decreased libido and sexual activity, decreased beard growth, and the “impression of having passed the zenith of life.” Data reported in standardized mean differences (SMDs) were interpreted as small (SMD, 0.2), medium (SMD, 0.5), and large (SMD, 0.8) effects (12).
Evidence to Recommendations
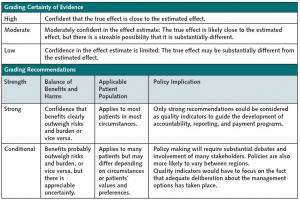
This guideline was developed by ACP’s CGC according to ACP’s guideline development process, details of which may be found in ACP’s methods paper (13). The CGC used the GRADE (Grading of Recommendations Assessment, Development and Evaluation) tables in the accompanying systematic review (12) when reporting the evidence and graded the recommendations using GRADE methodology (14, 15) (Figure 1). The accompanying GRADE Evidence to Decision tables illustrate the Evidence to Decision framework supporting the recommendations (Supplement 2).
Figure 1.
Grading the certainty of evidence and strength of recommendations of ACP clinical guidelines by using GRADE.
ACP = American College of Physicians; GRADE = Grading of Recommendations Assessment, Development and Evaluation.
The guideline underwent a peer review process through the journal and was posted online for comments from ACP Regents and ACP Governors, who represent internal medicine and its subspecialty physician members at the national and international levels.
CGC Public Panel Review
The development of this guideline also included perspectives, values, and preferences of 2 CGC members who represent the public and a 7-member CGC Public Panel, who rated outcomes and provided comments on the guideline.
Summary of the Evidence
The evidence review identified 38 randomized controlled trials that met the inclusion criteria to evaluate the benefits and harms of testosterone treatment. The mean age across all studies was 66 years, and follow-up ranged from 6 to 36 months. Participants in most studies had a mean baseline total testosterone level of 10.4 nmol/L (300 ng/dL) or lower (20 studies), although several studies included men with higher baseline testosterone levels. Outcomes did not vary substantially in studies that had different baseline testosterone levels or evaluated different testosterone formulations.
Quality of Life
Low-certainty evidence from 7 trials showed a small improvement in quality of life as measured by the AMS scale (SMD, 0.33 lower [95% CI, 0.50 to 0.16 lower]); however, this change may have been driven by improvement in sexual function, which is an AMS subscale.
Sexual Function
Moderate-certainty evidence from 7 trials showed a small improvement in global sexual function as measured by the IIEF or AMS scale (SMD, 0.35 higher [CI, 0.23 to 0.46 higher]).
Low-certainty evidence from 7 trials showed a small improvement in erectile function (SMD, 0.27 higher [CI, 0.09 to 0.44 higher]).
Physical Function
Low-certainty evidence from 7 trials showed little to no difference in physical function as assessed by objective measures, such as walk tests (SMD, 0.14 higher [CI, 0.02 to 0.27 higher]). Low-certainty evidence from 5 trials showed little to no difference in physical function from self-reported measures, such as the Short Form-36 Health Survey (SMD, 0.15 higher [CI, 0.19 lower to 0.50 higher]).
Adverse Cardiovascular Events
Low-certainty evidence from 14 trials showed a small increase to no difference in adverse cardiovascular events (Peto odds ratio, 1.22 [CI, 0.66 to 2.23]).
Serious Adverse Events
Moderate-certainty evidence from 8 trials found no evidence of increased risk for serious adverse events (Peto odds ratio, 0.94 [CI, 0.73 to 1.21]) or withdrawals due to adverse events with testosterone treatment (Peto odds ratio, 0.92 [CI, 0.65 to 1.28]).
Mortality
Although trials were not powered for mortality differences and many excluded men with a higher risk for death, including those with recent cardiovascular disease, pooled analysis of 12 studies showed fewer deaths among patients treated with testosterone than those who received placebo (incidence, 0.4% [CI, 0.07% to 0.99%] vs. 1.5% [CI, 0.48% to 2.89%]; Peto odds ratio, 0.47 [CI, 0.25 to 0.89]). The evidence review graded this evidence as low certainty. However, because of very serious imprecision, low event rates, and potential fragility of the results, the CGC felt that it had insufficient evidence to make conclusions about mortality.
Other Outcomes
Testosterone treatment improved vitality and fatigue by a less-than-small amount (3 trials) (SMD, 0.17 higher [CI, 0.01 to 0.32 higher]). Five trials assessed depressive symptoms by using various scales and showed a less-than-small improvement (SMD, 0.19 lower [CI, 0.32 to 0.05 lower]), although most men in the studies did not have baseline depression. No differences were found in cognitive function in studies that assessed it by using various scales. Few studies reported fractures, and they occurred too rarely to draw any conclusions about the effect of testosterone treatment.
Evidence from 20 observational studies with a mean follow-up ranging from 0.73 to 10.3 years showed no increased risk for mortality, cardiovascular events, prostate cancer, or pulmonary embolism or deep venous thrombosis. Evidence for long-term safety is lacking. Most studies excluded men with recent cardiovascular disease. No consistent differences were observed in harms according to transdermal versus intramuscular formulations in the included observational studies that addressed the comparison.
Values and Preferences
An additional, rapid review independent of the systematic evidence review identified 6 studies that assessed patient preferences (Supplement 1). Two studies assessed patient preferences among treatment options (16, 17). Fifty-three percent of patients receiving testosterone treatment (n = 382) chose injectable testosterone over gel-based pellet regimens, mostly because of lower cost (16). A second study (n = 54) analyzed preferences of a subpopulation of patients after they used a testosterone replacement therapy (TRT) product for more than 1 month and found that most (71%) preferred a topical gel over an injection or patch for reasons attributed to convenience, ease of use, and nonstaining of clothes, among others (17).
Regarding the relative importance of outcomes, 2 studies reported similar findings (18, 19). Among 312 men with hypogonadism, the most bothersome symptoms were lack of energy (90% of patients who elected to receive TRT vs. 81% who refused TRT; P = 0.075), decreased strength and endurance (86% vs. 76%; P = 0.077), and deterioration in work performance (52% vs. 31%; P = 0.004) (18). In a survey of 95 patients with hypogonadism who had not received TRT, the most bothersome symptoms were erectile dysfunction (66.3%), followed by decreased sex drive (55.8%) and loss of energy or increased tiredness (47.4%) (19).
Three studies evaluated men’s willingness to undergo treatment. In a study of 110 patients attending a urologic consultation (half of whom had a clinical diagnosis of hypogonadism), 43% of the respondents reported an interest in testosterone treatment, regardless of their urologic diagnosis, although half were unsure of the risks of testosterone treatment (20). Another study showed that erectile dysfunction was the most frequently reported reason (66%) for seeking treatment, followed by loss of energy or increased tiredness (59.0%) and decreased sex drive (57.9%) (19). However, TRT discontinuation rates between 30% and 62% have been reported (19, 21).
Costs
The annual cost in 2016 per beneficiary for TRT was $2135.32 for the transdermal and $156.24 for the intramuscular formulation, according to paid pharmaceutical claims provided in the 2016 Medicare Part D Drug Claims data.
Inconclusive Areas of Evidence
Because studies have had limited follow-up, evidence on long-term benefits or harms of testosterone treatment is lacking. Evidence about mortality also is inconclusive because of very serious imprecision, low event rates, and potential fragility of the results.
Multiple Chronic Conditions: Clinical Considerations
Results from trials that included only obese men or men with diabetes or metabolic syndrome were similar to those of the overall pooled analysis reported earlier.
Recommendations
Figure 2 summarizes the recommendations and clinical considerations.
Figure 2.
Summary of the ACP guideline on testosterone treatment in adult men with age-related low testosterone.
ACP = American College of Physicians. A downloadable version of this figure is available as a Supplement at Annals.org.
Recommendation 1a: ACP suggests that clinicians discuss whether to initiate testosterone treatment in men with age-related low testosterone with sexual dysfunction who want to improve sexual function (conditional recommendation; low-certainty evidence). The discussion should include the potential benefits, harms, costs, and patient’s preferences.
Recommendation 1b: ACP suggests that clinicians should reevaluate symptoms within 12 months and periodically thereafter. Clinicians should discontinue testosterone treatment in men with age-related low testosterone with sexual dysfunction in whom there is no improvement in sexual function (conditional recommendation; low-certainty evidence).
Recommendation 1c: ACP suggests that clinicians consider intramuscular rather than transdermal formulations when initiating testosterone treatment to improve sexual function in men with age-related low testosterone, as costs are considerably lower for the intramuscular formulation and clinical effectiveness and harms are similar.
Evidence shows that men with age-related low testosterone (testosterone threshold, ≤10.4 nmol/L [300 ng/dL]) (22, 23) may show small improvements in sexual functioning, as measured by the AMS scale, IIEF erectile dysfunction domain, or IIEF-5 (simplified 5-item IIEF) scale. However, evidence shows little to no improvement in physical function, depressive symptoms, energy and vitality, or cognition. Evidence for quality of life was limited to results from the AMS scale, and the small increase was probably driven by improvements in sexual function. Evidence for harms was difficult to judge because of the low power of studies or limited information available; thus, conclusions about the impact of testosterone therapy on cardiovascular health, prostate cancer, or other harms are difficult to make. The FDA requires labeling of testosterone products to include a warning about a possible increased risk for heart attacks and strokes. Evidence shows variability associated with patient values and preferences; thus, it does not support the use of testosterone treatment in all patients with age-related low testosterone without informed decision making.
Most studies included in the evidence review followed patients for 12 months or less, so the longer-term benefits and harms of testosterone treatment are unknown. Without an improvement in symptoms, the treatment would incur additional cost with no clear benefit. Patients’ symptoms should be reevaluated to determine whether treatment has been effective; if no benefit in sexual function occurs within 12 months, treatment should be discontinued.
Both intramuscular and transdermal testosterone applications have been associated with improvements in sexual function. Evidence from indirect comparisons suggests no substantial differences in clinical effectiveness, benefits, or harms between intramuscular and transdermal testosterone applications, although very little evidence exists from direct comparisons of the 2 formulations (Supplement 2) (13). Patients preferred the intramuscular formulation over transdermal applications because of cost (16) (Supplement 1). Because the 2 formulations are similar in terms of benefits and harms but the intramuscular formulation is substantially cheaper ($156.32 vs. $2135.32 per person per year for the transdermal option), the intramuscular application is the preferred testosterone treatment.
Recommendation 2: ACP suggests that clinicians not initiate testosterone treatment in men with age-related low testosterone to improve energy, vitality, physical function, or cognition (conditional recommendation; low-certainty evidence).
Evidence showed very little or no benefits for common concerns of aging, including energy and vitality, physical function, and cognition. In addition, evidence on long-term harms is lacking. Although the evidence review found a decrease in mortality with testosterone treatment, studies were not powered to detect mortality differences, few events occurred, the evidence had serious imprecision, the results were potentially fragile, and studies excluded men at greatest risk for dying. Thus, the CGC feels that evidence is insufficient to draw definitive conclusions about the effect of testosterone treatment on mortality. Therefore, men with age-related low testosterone should not be prescribed testosterone treatment unless its purpose is to treat sexual function issues.
Appendix: Detailed Methods
The Minnesota Evidence-based Practice Center conducted the supporting evidence review. Details of the ACP guideline development process may be found in the ACP methods paper (13). Disclosure of interests and management of any conflicts may be found at www.acponline.org/clinical_information/guidelines/guidelines/conflicts_cgc.htm.
Key Question Addressed
What are the benefits and harms of testosterone therapy for men? Are the benefits and harms affected by:
- Baseline testosterone level
- Baseline symptoms
- Age
- Comorbid conditions (such as obesity, opiate use, corticosteroid use, HIV)
- Targeted or achieved testosterone level
Search Strategy
Reviewers searched several databases for studies and systematic reviews published in English from 1980 to May 2019.
Quality Assessment
Reviewers assessed risk of bias by using a modified Cochrane approach (24) for randomized controlled trials and a modified Agency for Healthcare Research and Quality approach for observational studies (25).
Population Studied
Adult men.
Interventions Evaluated
Transdermal and intramuscular testosterone treatments approved by the FDA.
Comparator
No testosterone treatment, including placebo.
Outcomes
Both clinician and nonclinician public members were asked to independently rate the importance of evaluated outcomes. Physician members rated serious adverse events the highest, whereas public members rated cardiac adverse events the highest. Quality of life; erectile function; cognitive function; and harms, including serious adverse events, major adverse cardiovascular events, deep venous thrombosis or pulmonary embolism, mortality, and prostate cancer, were rated as critical outcomes. Energy and vitality, physical function, mood (depression), fracture reduction, libido, and lower urinary tract symptoms were rated as important outcomes.
Timing
Treatment for 6 months or longer.
Setting
Outpatient.
Target Audience
All clinicians.
Target Patient Population
Adult men.
Public and Patient Involvement
The development of this guideline also included perspectives, values, and preferences of 2 nonphysician CGC members who represent the public and a 7-member CGC Public Panel.
Peer Review
The supporting evidence review and guideline each were peer reviewed through the journal. The guideline was posted online for comments from ACP Regents and ACP Governors, who represent internal medicine and its subspecialty physician members at the national and international levels.
References
- TajarA, FortiG, O’NeillTW, et alEMAS GroupCharacteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study.J Clin Endocrinol Metab20109518108 CrossRef PubMed
- FeldmanHA, LongcopeC, DerbyCA, et alAge trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study.J Clin Endocrinol Metab20028758998
- NieschlagE, SwerdloffR, BehreHM, et alInvestigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, and EAU recommendations.Int J Androl2005281257
- HarmanSM, MetterEJ, TobinJD, et alBaltimore Longitudinal Study of AgingLongitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging.J Clin Endocrinol Metab20018672431
- StanworthRD, JonesTH. Testosterone for the aging male; current evidence and recommended practice.Clin Interv Aging200832544
- MaggiM, SchulmanC, QuintonR, et alThe burden of testosterone deficiency syndrome in adult men: economic and quality-of-life impact.J Sex Med20074105669
- MulliganT, FrickMF, ZurawQC, et alPrevalence of hypogonadism in males aged at least 45 years: the HIM study.Int J Clin Pract2006607629
- PetakSM, NankinHR, SparkRF, et alAmerican Association of Clinical EndocrinologistsAmerican Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients—2002 update.Endocr Pract2002 Nov-Dec844056
- WuFC, TajarA, BeynonJM, et alEMAS GroupIdentification of late-onset hypogonadism in middle-aged and elderly men.N Engl J Med201036312335
- BassilN, MorleyJE. Late-life onset hypogonadism: a review.Clin Geriatr Med201026197222